Knowledge
The discovery of austenite, ferrite and martensite and their historical significance
From Experience to Science - The Beginning of Steel
The utilization of steel by humans can be traced back to the Iron Age of the 12th century BC. At that time, people discovered through experience that prolonging the heating time of iron ore in a charcoal furnace would make the iron harder, and they developed the quenching hardening technique. Ancient India had mastered the technology of producing Uzh steel in the 3rd century BC. This is a type of crucible steel with a carbon content between pig iron and wrought iron, and its outstanding performance enabled it to be exported all over the world. However, these early steel production methods mostly relied on generations of passed-down experience, and the underlying scientific principles remained shrouded in mystery.
Entering the 19th century, the vigorous rise of the Industrial Revolution in Europe and North America led to an unprecedented demand for high-performance steel materials. The rapid development of railways, steam engines, and large machinery made the traditional empirical production methods unable to meet the urgent requirements for standardized and precise control of material properties. In 1802, people began to explore the carbon content in steel, and in 1827 to 1829, graphite was confirmed to be pure carbon, marking the beginning of scientific research on iron-carbon alloys. The macroscopic properties of steel are closely related to its microscopic crystal structure. Industrial demands prompted scientists to shift from empirical methods to principle-based exploration. Although ancient craftsmen discovered through experience that quenching could make steel harder, they could not explain the underlying mechanism. The huge driving force of the Industrial Revolution compelled scientists to answer core questions such as "why does steel become harder" and "how to precisely control its properties". This pursuit of "knowing what is the case" to "knowing why it is so" eventually gave rise to the discovery of key microscopic phases such as ferrite, austenite, and martensite, and gradually constructed the iron-carbon phase diagram to describe the laws of these phase transformations, thereby elevating steel smelting to a rigorous science.
The Pioneer of the Microscopic World - The Discovery and Naming of Ferrite
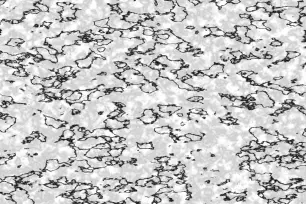
British scientist Henry Clifton Sorby (1826-1908) is hailed as the "father of metallography". He creatively applied the technique of preparing thin slices of rocks (including the use of polarized light microscopes) to opaque steel samples. Sorby began systematic research on the microstructure of steel in 1863 and, through acid etching technology, was the first to observe the internal structure of steel under a microscope. This work revealed the influence of carbon content on the strength of steel. Although the research results were completed between 1863 and 1865, they were not published until 1886-1887.
Sorby's microscopic observations revealed the crystal structure of pure iron, which was later named "Ferrite" (derived from the Latin word "ferrum", meaning "iron"). In metallurgy, ferrite specifically refers to the pure iron phase and has a body-centered cubic (BCC) crystal structure. Ferrite is the stable form of iron at room temperature, with a very low carbon solubility, approximately 0.025% at the highest. It is magnetic, relatively soft, and has good ductility but low strength. Sorby introduced microscopes to steel research, pioneering metallography and enabling people to "see" the internal structure of steel, laying the foundation for subsequent phase transformation studies.
2. Unveiling the Secret of High Temperature - The Birth and Naming of Austenite
To meet the increasing demands of the industrial sector for the performance of steel, scientists began to draw diagrams that describe the distribution of phase regions of iron-carbon alloys at different temperatures and carbon contents. British metallurgist William Chandler Roberts-Austen (1843-1902) proposed the first relatively complete iron-carbon temperature-concentration diagram (T-x diagram) in 1897, and is considered the pioneer of the modern iron-carbon phase diagram. He used Henry Louis Le Chatelier's rhodium thermocouple and his own designed temperature recording equipment to systematically study the phase transformation critical points during the heating and cooling process of iron-carbon alloys, revealing the special crystal structure of iron that can dissolve more carbon at high temperatures.
In honor of Roberts-Austen's contribution, this special crystal structure of iron was named "austenite". Austenite is the stable phase of iron-carbon alloys at high temperatures, with a face-centered cubic crystal structure. It can dissolve more carbon, up to 2%, and is non-magnetic. It has good plasticity and ductility at high temperatures. When austenite cools, it will transform into ferrite or other phases, but by adding alloy elements such as nickel and manganese, austenite can remain stable at room temperature (such as austenitic stainless steel).
At the same time, Roberts-Austen's iron-carbon phase diagram is also an important milestone in materials science, providing a systematic and quantitative framework to understand the phase transformation behavior of iron-carbon alloys. This transition from "what is" to "what will happen" enables the heat treatment process of steel to shift from trial-and-error methods to precise control based on scientific principles, significantly improving the controllability and reliability of material performance, and is the cornerstone of modern materials engineering.
3. The Miracle of Quenching - The Appearance and Naming of Martensite
As early as the Iron Age, artisans had discovered that rapid cooling could increase the hardness of iron and applied this principle to the manufacture of tools and weapons. However, the underlying mechanism remained a mystery.
German metallurgist Adolf Martens (1850-1914) was a pioneer in the field of materials engineering. In the 1880s (around 1880), he first identified a specific crystal structure in quenched steel using an optical microscope. This structure forms when steel is rapidly cooled (quenched) from extremely high temperatures. He conducted in-depth research on the microstructure of steel and provided crucial microscopic evidence for understanding the phenomenon of quench hardening.
In honor of Adolf Martens, in 1895, French scientist Floris Osmond named this crystal structure formed through quenching "Martensite". Martensite is a supersaturated solid solution of carbon in α-Fe and has a body-centered tetragonal (BCT) crystal structure. Martensite is renowned for its extremely high hardness and strength, with hardness mainly depending on the carbon content. However, high hardness is accompanied by higher brittleness. The formation of martensite is a non-diffusive phase transition, where carbon atoms are "trapped" in the lattice during the rapid cooling process and cannot diffuse to form carbides. The discovery and understanding of martensite are key to modern high-performance steel manufacturing. By controlling the quenching process, engineers can precisely form martensite to achieve the desired hardness and strength, and it is widely used in tools, tools, aerospace, and medical equipment, among other fields.
4.The Interconnection of the Three Major Forces and the Industrial Transformation
Sobie's identification of ferrite, Roberts-Austin's discovery of austenite and the drawing of phase diagrams, as well as Martens' observation and naming of martensite, jointly formed the theoretical basis of the modern iron-carbon phase diagram. The iron-carbon phase diagram clearly depicts the stable regions of iron, austenite, ferrite, cementite (Fe₃C), and other phases at different temperatures and carbon contents, as well as their mutual transformation relationships. At the beginning of the 20th century, the scientific community promoted the unification of phase transformation names, and in 1909-1911, the sixth congress of the International Materials Testing Association reached a consensus on the unification of phase transformation names.
During the steel heat treatment process, ferrite, austenite, and martensite exhibit complex interactions. Austenite is usually the starting point of heat treatment. Heating steel to the austenite region (austenitization) allows carbon atoms to fully dissolve and form a uniform solid solution. The subsequent cooling path determines the final microstructure:
● Slow cooling: Carbon atoms have sufficient time to diffuse, forming a layered structure composed of ferrite and cementite, known as pearlite.
● Rapid cooling (quenching): Austenite is rapidly cooled below the critical temperature, and carbon atoms have no time to diffuse, resulting in a shear deformation of the face-centered cubic lattice, forming a supersaturated body-centered tetragonal martensite, achieving extremely high hardness.
Although the iron-carbon phase diagram is the foundation for understanding the behavior of iron-carbon alloys, other alloying elements in actual alloy steels significantly alter the phase transformation temperatures, phase zone boundaries, and final properties. For example, nickel and manganese can stabilize austenite up to room temperature, forming austenitic stainless steel, which has excellent corrosion resistance, plasticity, and high and low-temperature mechanical properties. Ferrite stainless steel is known for its magnetic properties, ductility, and limited corrosion resistance. A deep understanding of these basic phases enables modern metallurgists to design more complex, custom-performance multi-phase steels to meet higher strength and ductility requirements.
5.Eternal Legacy - Insights for Materials Science and Engineering
From the ancient craftsmanship of smelting steel based on experience to the revelation of the microscopic mysteries of steel by scientists in the 19th century, the discoveries of ferrite, austenite and martensite represent a significant leap in human understanding of materials. These milestones collectively laid the foundation for modern physical metallurgy.
These three basic phases and their interrelationships constitute the core of modern physical metallurgy and form the basis for understanding and designing almost all iron-based alloys. They guide traditional heat treatment processes and also inspire the development of a new generation of high-performance materials. To this day, in-depth research on these phases continues, driving material innovation and optimization. The lasting value and far-reaching impact of these fundamental scientific discoveries continue to drive interdisciplinary innovation and technological progress.